New Delhi: Indian Scientists have discovered a strategy to synthesize novel solid adsorbents for CO2 capture and utilization.
Carbon capture and utilization are growing fields of research focusing on reducing CO2 emissions. Although several industrial advancements have already been demonstrated, none of the technologies can provide an economically viable and complete CO2 capture and utilization solution. Therefore, fundamental research on novel solid adsorbents might offer a critical material for CO2 capture and CO2 utilization.
Professor Rahul Banerjee’s group at IISER-Kolkata, with support from Department of Science & Technology, Govt. of India under Mission Innovation program, has demonstrated a strategy to synthesize novel solid adsorbents, especially for CO2 capture and CO2 utilization. Prof. Banerjee’s group has discovered special types of nanoparticles or microparticles which can capture CO2 in their micro and mesoporous voids.
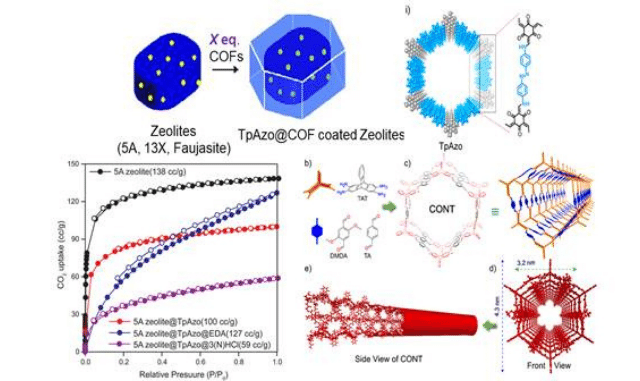
The novel materials with distinct physical properties on its surfaces that have been synthesized include porous Covalent organic frameworks like COF-graphene Janus thin films published in ‘Journal of American Chemical Society’ porous covalent bonded organic nanotubes published in Nature Chemistry, and COF coated zeolite published in ‘Journal of American Chemical Society’.
The judicious choice of 2D graphene sheets as a grafter helped the researchers to design and create COF-graphene Janus thin films through the interactions (non-covalent) between the COF and graphene, rendering flexible porous Janus films at the DCM-water interface. The newly designed COF-coated zeolites could be an excellent candidate for CO2 storage in the industry due to their high surface area and increased chemical stability.
The high CO2 uptake for the COF coated zeolites, even after treatment with weak acids makes it appropriate for industrial purposes. The COFs coating prevented the degradation of zeolite structure from moisture, weak acids, and water. The CO2 uptake data for COF coated zeolite at 1 bar, 293K is 132 cc/g, supersedes the CO2 uptake data of zeolite under the same condition.
Rahul Banerjee’s group has recently discovered purely covalent bonded organic nanotubes (CONTs) with a hitherto unavailable structure via a novel bottom-up approach. Although zero-dimensional covalent organic cages and two- and three-dimensional covalent organic frameworks were previously reported, the synthesis of one-dimensional organic nanotubes was hitherto unheard of. The synthesized CONTs have the edge over the analogous carbon nanotubes (CNTs) in functionalization, synthetic conditions, and porosity which exhibits a BET surface area of 321 m2 g-1. They are also promising candidates for the efficient CO2 adsorption with a CO2 uptake capacity of 60-80 cc g-1 at 1 bar and 293 K. These CONTs have also showcased photosensitizing ability, which can convert the adsorbed CO2 into CO (130-200 µmol g-1 h-1) upon irradiation of visible light (400-700 nm).