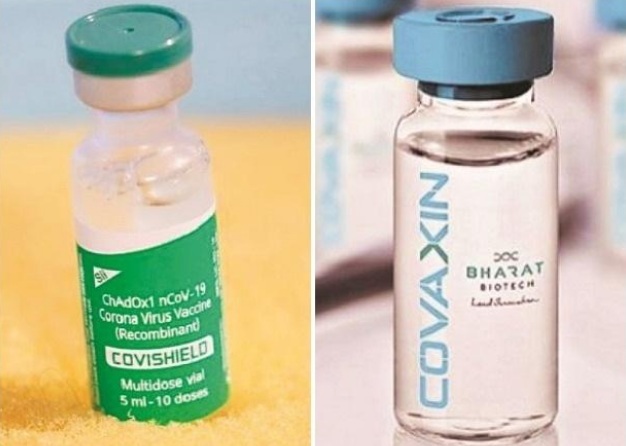
New Delhi: A mix of Covishield and Covaxin, the two main vaccines of India’s Covid vaccination programme, can actually yield better results, revealed the Indian Council of Medical Research.
A study by ICMR involving 98 people, 18 of whom had inadvertently received Covishield as first dose and Covaxin as the second in Uttar Pradesh showed that combining these two COVID-19 vaccines elicited better immunogenicity than two doses of the same vaccine.
The study also found that immunisation with a combination of Covishield and Covaxin was safe and the adverse effects were also found to be similar when compared to the same dose regimen.
The study titled Serendipitous COVID-19 Vaccine-Mix in Uttar Pradesh, India: Safety and Immunogenicity Assessment of a Heterologous Regime’ has been uploaded on medRxiv, a preprint server and is yet to be peer-reviewed.
“To the best of our knowledge, this is the first study which reports the effects of heterologous prime-boost vaccination with an adenovirus vectored vaccine followed by an inactivated whole virus vaccine,” the researchers said.
The study was only conducted on 18 people of Uttar Pradesh’s Siddharth Nagar, who by mistake received two doses of two separate vaccines. According to the finding of the study which is yet to be peer-reviewed immunisation with a combination of an adenovirus vector platform-based vaccine, followed by inactivated whole virus vaccine was not only safe but also elicited better immunogenicity.
Covishield, manufactured by Pune’s Serum Institute of India, is the adenovirus vector platform-based vaccine and Covaxin, developed and manufactured by Bharat Biotech and the ICMR, is the whole virus vaccine. Covishield and Covaxin belong to two different types.
Mixing of vaccines is being discussed globally all studies are in favour of mixing two vaccines to increase the protection against future infection. The subject expert committee of the Central Drugs Standard Control Organisation has in July recommended a trial of mixing Covishield and Covaxin, which will be conducted by the Christian Medical College in Vellore on 300 healthy volunteers.