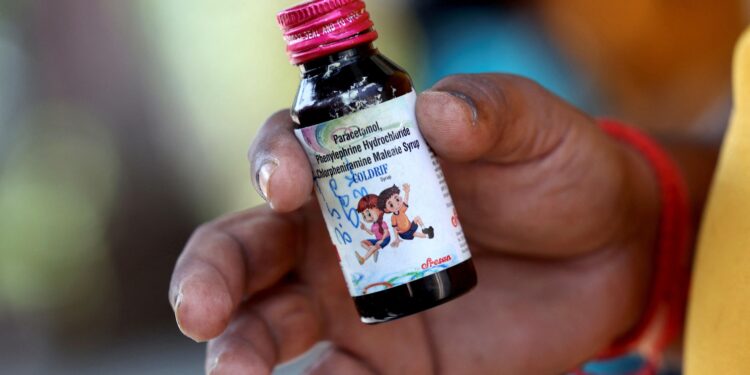
The World Health Organization (WHO) has issued a warning about three contaminated cough syrups found in India, weeks after the deaths of several children in Madhya Pradesh.
Among them is Coldrif syrup, which recently drew national attention following the tragedy. The WHO has asked all countries to report if they detect any of these syrups in their markets.
WHO’s Warning
According to reports, the WHO has identified these syrups:
-
Coldrif (Sresan Pharmaceuticals, Tamil Nadu)
-
Respifresh TR (Rednex Pharmaceuticals)
-
ReLife (Shape Pharma)
The health agency said these medicines may cause severe and potentially life-threatening illness.
Toxic Substance Found
Tests on Coldrif samples revealed the presence of diethylene glycol (DEG) — a toxic chemical historically linked to mass poisonings.
-
The DEG level in the syrup was nearly 500 times higher than the safe limit.
-
Most victims were children from Parasia village in Chhindwara, Madhya Pradesh.
Following the discovery, the Tamil Nadu-based Sresan Pharmaceuticals had its manufacturing license revoked, and its owner G. Ranganathan was arrested.
Indian Authorities Respond
India’s Central Drugs Standard Control Organization (CDSCO) told the WHO that the syrups were not exported outside India.
The US also confirmed that no contaminated cough syrups were shipped there.
Deaths Trigger National Outrage
At least 22 children under the age of five died after consuming the Coldrif syrup. The health ministry has now urged doctors to avoid prescribing cough syrups to children below five years, especially those under two.
Officials have ordered inspections of other drug factories across Tamil Nadu and nearby states to ensure compliance with safety standards.